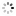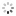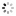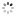Important Collectors Wristwatches, P...
Geneva, Mandarin Oriental Hotel Du Rhône, Oct 15, 2006
LOT 26
"A Madame la Comtesse de Laval" Breguet, No. 3537, sold to the Comtesse de Laval on July 17, 1821, for 1,180 francs. Very fine and extremely rare, platinum and yellow gold pocket watch with ruby cylinder escapement.
CHF 25,000 - 30,000
EUR 16,000 - 20,000 / USD 20,000 - 25,000
Sold: CHF 82,600
C. Four-body, "forme collier", polished, coin-edge band, yellow gold bezel, border and bow. Hinged platinum cuvette. D. Matte silver, engine-turned, brushed chapter ring with radial Roman numerals, outer minute chapter with dot indexes, eccentric subsidiary seconds between 4 and 5 o'clock. Blued steel "Breguet" hands. M. 44 mm., 20''', simple caliber, standing barrel, overhanging ruby cylinder escapement, plain three-arm balance with parachute on the top pivot, blued steel flat balance spring, index regulator with bimetallic temperature compensation curb. Dial and cuvette signed. Diam. 52 mm.
| Grading System | |
|---|---|
Grade: |
|
Case: 3 |
Good |
Movement: 3* |
Good Overhaul recommended, at buyer's expense |
Dial: 3-01 |
Good HANDS Original |
Notes
Countess Alexandrina Grigorievna Laval (1772 - 1850)
Alexandrina Grigorievna was born on March 18, 1772, into a family used to displaying its wealth with pride. Her father was Grigori Vassilievitch Kozitsky, Secretary of State of Tsarina Catherine II; her mother was one of four daughters of the famous millionaire Ivan Semenovitch Miasnikoff, and her dowry included 19,000 peasants, a large sum of money, numerous precious objects, and factories in the Urals. At the age of twenty-five, Alexandrina Grigorievna received a marriage proposal from a French expatriate by the name of Laval. Unfortunately for her, the family?s youngest daughter had wed an aristocrat, Prince A. Belosselsky-Belozersky, and her mother firmly vetoed the proposal. Deeply in love with Laval, Alexandrina Grigorievna decided her happiness was worth fighting for. Accordingly, she wrote to the Tsar himself, who immediately demanded an explanation from the unwilling mother. Mrs. Kozitsky justified her refusal by claiming that ?Laval is not of our religion, comes from ?God knows where? and hasn?t a sufficiently high social standing?. Paul I, upon hearing these explanations, laconically ruled that the mrriage should proceed: ?He is a Christian, I know him, and his standing is quite high enough for a Kozitsky?. The ceremony took place in the parish on the very next day, in the year 1799, without any preparation whatsoever. The fiancee brought with her an impressive dowry which contained, amongst other riches, the Voskrenssky factory in the Urals. Before she died in 1833, Mrs. Kozitsky made up somewhat for her earlier hesitations, by generously giving a number of properties to the Lavals, ?given that they are less wealthy than Princess Belosselsky?. Countess Laval had one son (Vladimir), who joined the Imperial Guard, and four daughters (Katacha, Zenaïda, Sophia, and Alexandrina). She died on November 17th, 1850, and was buried in the Saint-Lazare cemetery of the Alexander Nevsky monastery. Prince P. Dolgourouky?s writings are full of praise for Countess Laval: for her tact, her firmness, but also for her common sense and lively character. She was also well educated, displaying keen interest in the arts. In her residence on the ?Quai Anglais?, she frequently received poets, writers, and art connoisseurs, together with members of the nobility; Prince Viazemski and Turgenieff were frequent visitors, and she was a close friend of Madame de Staël.
Platinum
Platinum is a beautiful, silvery-white precious metal that is both malleable and ductile. Its symbol is Pt, its specific gravity 21.5, and its melting point is 1773 degrees. Platinum will not oxidize in air at any temperature, though it is corroded by halogens, cyanides, sulfur, and caustic alkalis. It is insoluble in hydrochloric and nitric acid, but dissolves when they are mixed as aqua regia, forming chloroplatinic acid. Platinum is rarer, heavier, and 11% denser than gold. One cubic foot weighs approximately 1330 pounds. The ancient Egyptians employed gold containing traces of platinum from the ancient kingdom of Nubia, which they used to create jewelry and adornments. A magnificent sarcophagus dating from 700 BC, made for the high priestess Shepenupet, daughter of the King of Thebes, was decorated with gold and platinum hieroglyphics, and a small platinum document casket was placed in her tomb. Ancient South American civilizations, including the Incas, used platinum and gold for nose rings and other types of ceremonial jewelry. The metal was subsequently forgotten for thousands of years, perhaps due to the attitude of the Conquistadors, who, thirsty for gold, considered it an inferior form of silver, and threw it back into the rivers of Ecuador to ?ripen?. The name ?platinum? derives in fact from the derogatory Spanish ?platina?, meaning ?little silver?. The metal was ?rediscovered? in 1735, in the gold-bearing sands of Colombia, along with gold and diamonds, in the debris of ancient rocks. In the early years of the 19th century, British scientist William Hyde Wollaston obtained the first pure sample. When the means of making platinum malleable was discovered, its commercial uses began to multiply. The metal is so flexible that one gram of it can be drawn to produce a thread over one mile long. Platinum has many industrial uses; much more so than either silver or gold. It also has excellent conductivity and many applications in electronics and medicine. For these reasons, platinum is used for modern-day pacemakers. Breguet was probably the first watchmaker to use platinum for cases (see Antiquorum's ?The Art of Breguet? sale, April 14, 1991, for a platinum and gold-cased watch sold in 1822, lot 73), being followed by the Genevan horologist Bautte in the 1830s. Breguet also used platinum for the oscillating weights of self-winding watches, and for balance screws and balances, because its specific gravity afforded greater inertia than other metals. Jewelers have long appreciated platinum, both for its technical characteristics - exceptional hardness, a high melting point, and the fact that it does not tarnish and can take a high polish - as well as for its esthetic qualities. The beauty of its silvery-white sheen made it a perfect complement to precious stones, and particularly diamonds. Cartier, in particular, made much of platinum?s decorative qualities, employing the metal in his famous ?garland style?, in which platinum enhanced the brilliance of diamonds to great effect. Indeed, Cartier proudly claimed to have been the first jeweler to use platinum. For a long time, the metal was more closely associated with science and industry than with jewelry. In France, platinum had shared a metal mark with gold, and was not recognized as a precious metal in its own right until April 1910, when it was given its own characteristic metal guarantee mark. In Switzerland platinum received a characteristic mark in February 1914. Today, platinum is considered by many to be the most exclusive of all the precious metals, because of its rarity and the difficulty with which it is fashioned.